Product Name: Salcaprozate Sodium
Alias: SNAC; Salcaprozate Sodium; Salcaprozate sodium; Salcaprozate Sodium (SNAC); sodium 8-(2-hydroxybenzamido)octanoate; sodium,8-[(2-hydroxybenzoyl)amino]octanoate; SODIUM 8-(2-HYDROXYBENZAMIDO)OCTANOATE (SNAC); SNAC,sodium 8-[(2-hydroxybenzoyl)amino]octanoate; Octanoic acid, 8-[(2-hydroxybenzoyl)amino]-, monosodium salt
CAS No.: 203787-91-1
EINECS No.: 231-019-4
Chemical Formula: C15H20NNaO4
Molecular Weight: 301.31
Chemical Structural Formula:
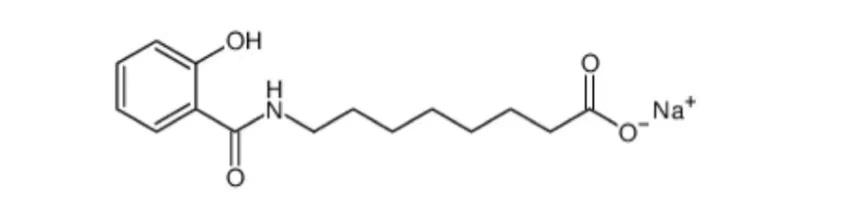
Description:
| Testing Items |
Specifications |
| [Characteristic] |
Appearance |
White to yellowish or pinkish crystalline powder |
| [Identification] |
HPLC |
The retention time of the main peak of the Sample solution corresponds to that of the Standard solution |
| UV |
The maximum absorption should be at the wavelength of 301nm and 238nm, and the minimum absorption should be at the wavelength of 228nm |
| IR |
Corresponding to Cholesterol CRS |
| [Inspection]
|
Relevant Substances |
Impurity A: ≤0.15% |
| Impurity E: ≤0.15% |
| Impurity G: ≤0.5% |
| The maximum unknown single impurity: ≤0.1% |
| Total impurities: ≤1.0% |
| Water |
≤2.0% |
| Heavy Metals |
≤20ppm |
| Arsenate |
≤0.0002% |
| Residual Solvents |
Methyl Alcohol: ≤0.5% |
| Isopropanol: ≤0.5% |
| DMF: ≤0.088% |
| Ethyl Alcohol: ≤0.5% |
| Benzene: ≤0.0002% |
| Microbial Contamination |
TAMC: ≤1000CFU/g |
| TYMC: ≤100CFU/g |
| E. coli should not be detected per 1g |
|
Residue on Ignition
|
≤0.1%w/w |
| [Assay] |
Sodium |
7.1% ~ 8.1%(On Dried Basis) |
| Salcaprozate Sodium |
98.0% ~ 102.0%(On Dried Basis) |

Storage Conditions:
Kept at 2℃~8℃ under an inert atmosphere.
For reducing the absorption of the moisture, it should be slowly warmed to ambient temperature before opened.
Application:
SNAC is an absorption enhancer for dicarbonate phosphate compounds, which is used for treating gastrointestinal diseases, especially for gastrointestinal diseases caused by malabsorption of dicarbonate phosphate compounds.